Electronic medication management
Digital health systems such as electronic medication management (EMM) can improve the safety and quality of health care.
By using EMM, healthcare services can reduce the number of preventable adverse medication events, and medication prescribing and dispensing errors. EMM systems can improve the accuracy, visibility and legibility of medical information, so that the communication between professionals and consumers is clearer.
EMM can apply to:
- Prescribing systems, such as general practitioner desktop systems or hospital clinical information systems that have electronic ordering
- Decision support systems, such as evidence-based order sets, allergy checking and medicine interactions
- Dispensing systems, such as pharmacy software and automated dispensing systems
- Ordering and supply solutions, such as the electronic transfer of prescriptions (ETP) and inventory solutions
- Electronic medical records.
Resources
The Commission has developed a number of resources to support EMM.
EMM is supported through the consistent and standardised presentation of medicines information and the [former] Australian Health Ministers' Advisory Council (AHMAC) endorsed the following guidelines for this purpose:
The National Guidelines for On-Screen Display of Medicines Information provide an evidence-based approach to maximise patient safety.
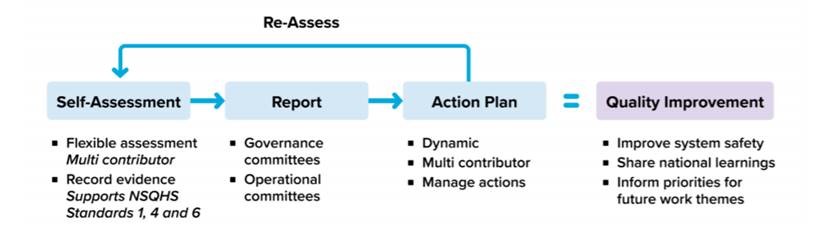
EMM Self Assessment Tool
To help health services independently assess the status of their EMM system implementation, the Commission has developed the EMM self-assessment tool (EMM SAT) online. The tool facilitates multi-contributor self-assessment, standardised reporting and quality improvement action plans.
For more information about the domains and indicators used in the tool please review the Electronic Medication Management Self-Assessment Domain and Indicators Summary.
Business requirements
The Electronic Medication Management Systems Business Requirements (the Requirements) are an annexe to the Electronic Medication Management Systems: A guide to safe implementation (third edition), published by the Commission in 2019.
The Requirements aim to support health services procure and assess EMM systems.
History
The Requirements were brought together from published and unpublished information sources and validated by an expert panel. The Commission has incorporated other EMM business requirements arising from the Australian context, including medication safety priorities, technical standards and national infrastructure initiatives.
The following literature reviews informed the development of the guide and the Requirements:
![]()
Electronic medication charts
Electronic medication charts allow safe and accountable medication management.


