The Atlas series examines variation in use of some commonly prescribed medicines. Medicines are effective when used appropriately for the right duration. However, substantial variation in use can suggest that some individuals and the community are being exposed to avoidable harms and unnecessary costs, while others may be missing out on an effective treatment.
Quality statement 8
A patient’s PIVC and insertion site is inspected by a clinician for signs of complications at least once per shift or every eight hours, when accessing the device, and if the patient raises concerns. Standard precautions including aseptic technique are used when performing site care and accessing the PIVC. Patency is checked and flushing is performed at intervals according to local policy to assess device function and minimise risk of device failure.
The Commission has developed new training pathways for Hand Hygiene Auditors (previously called General Auditors) and Hand Hygiene Auditor Educators (previously called Gold Standard Auditors).
Annual revalidation is a method of ensuring all Hand Hygiene Auditors and Hand Hygiene Auditor Educators remain up to date with their knowledge of the 5 Moments and audit practices. This ensures valid and reliable data for the National Hand Hygiene Initiative (NHHI).
Hand Hygiene Auditors (previously called General Auditors) conduct direct observational audits of healthcare worker hand hygiene compliance.
This page provides information on Hand Hygiene Auditor Educator Training.
Quality statement 4
A patient requiring a PIVC is assessed to identify the most suitable insertion site and PIVC (length and gauge) to meet their clinical needs and preferences for its location.
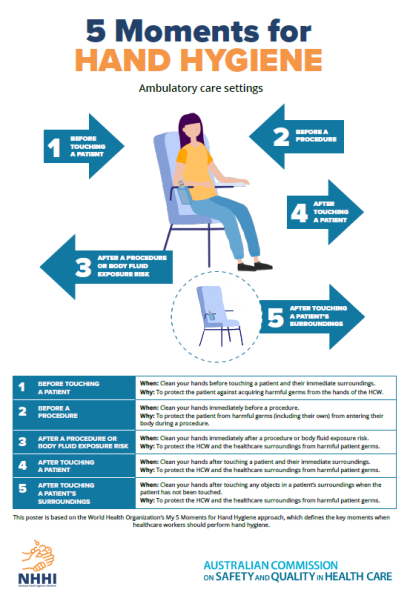
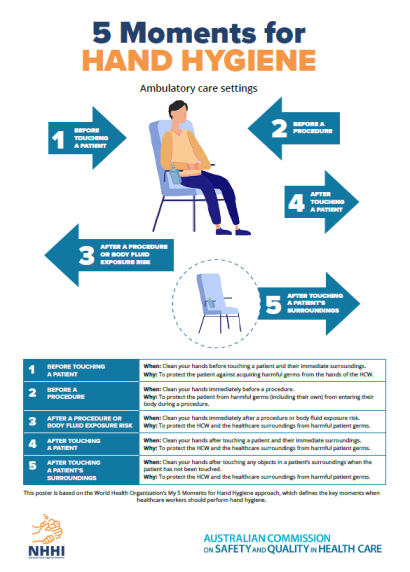
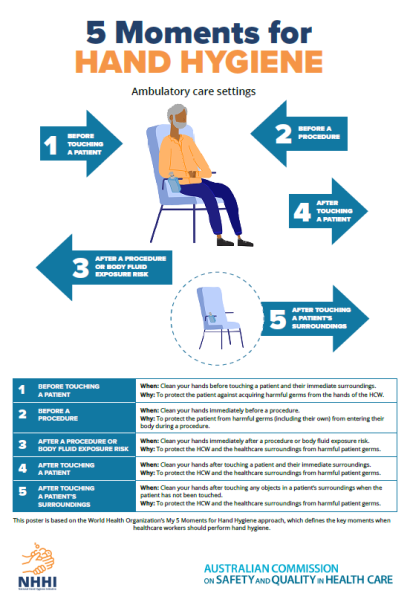
Hand hygiene compliance is assessed against a national benchmark, which is currently 80%. There are three national hand hygiene audits conducted each year:
- Audit period 1: 1 November to 31 March
- Audit period 2: 1 April to 30 June
- Audit period 3: 1 July to 31 October