The Commission delivered consultations to develop the requirements in partnership with all jurisdictions via the Clinical Trials Project Reference Group. A new report revealed broad support and a sense of genuine enthusiasm and anticipation for the proposed National One Stop Shop.
The Commission’s Data Governance Framework specifies the obligations of the Commission with regard to data acquisition, maintenance, sharing/permissions, reporting and publication.
This document provides an overview of the procedures for the operation of CARAlert and describes the roles and responsibilities of stakeholders.
Why your views are important
The Management of Peripheral Intravenous Catheters Clinical Care Standard was launched via webcast on Wednesday, 26 May 2021.
The slides from the event are available below.
This fact sheet provides users with information on the alignment of the actions and criteria from the 2017 Preventing and Controlling Healthcare-Associated Infections Standard (2017 Standard) to the 2021 Preventing and Controlling Infections Standard (2021 Standard).
On 26 May 2021, the Commission released the Management of Peripheral Intravenous Catheters Clinical Care Standard.
This rapid literature and evidence review was commissioned during development of the Sepsis Clinical Care Standard.
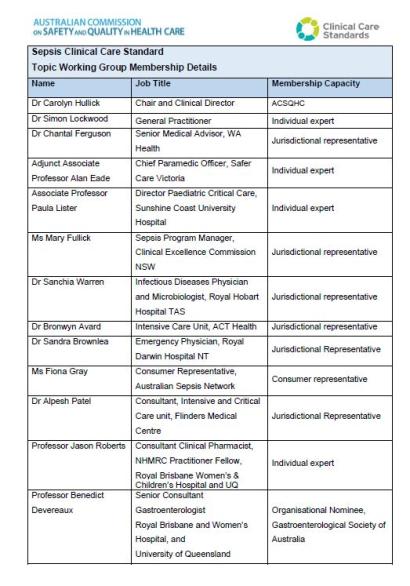
A list of members for the Sepsis Clinical Care Standard Topic Working Group.