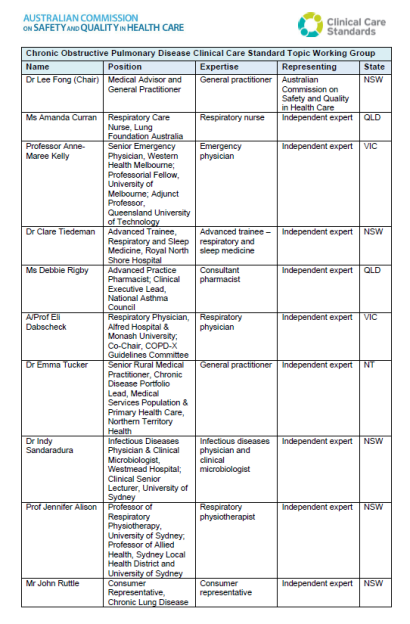
List of Topic Working Group members for the Chronic Obstructive Pulmonary Diseases Clinical Care Standard.
Download the standard
The Commission has released a new Advisory DI 23/01: Revised Diagnostic Reference Levels (DRLs) for Nuclear Medicine and Positron Emission Tomography.
On the Radar Issue 610 is now available.
Share your feedback on the draft Requirements for Cervical Screening before Friday 14 July.
Hear from Illawarra Shoalhaven Local Health District (ISLHD) about their journey to become a health literate organisation.
National Medicines Symposium - Wednesday, 8 November.
The 2022 Annual Report provides the results of analyses of data on confirmed critical antimicrobial resistances (CARs) submitted to the National Alert System for Critical Antimicrobial Resistances (CARAlert) for 2022, and trend data from 2017.
On the Radar Issue 609 is now available.
Presentation on short notice assessment for accreditation to the NSQHS Standards.