The Australian Group on Antimicrobial Resistance (AGAR) monitors AMR, epidemiologic and genetic patterns in bloodstream infections in Australia relating to three main organisms: Staphylococcus aureus, enterococcal species and gram-negative organisms. The 2020 AGAR Sepsis Outcome Programs Report provides analyses of data on AMR associated with episodes of bacteraemia, reported by 30 laboratories servicing 49 public and private institutions across Australia in 2020.
Update 25 provides data submitted to CARAlert for the reporting period 1 September to 31 October 2021 to support prevention and containment of antimicrobial resistance.
The tables below outline the circumstances where it may not be necessary to implement individual actions of the Primary and Community Healthcare Standards.
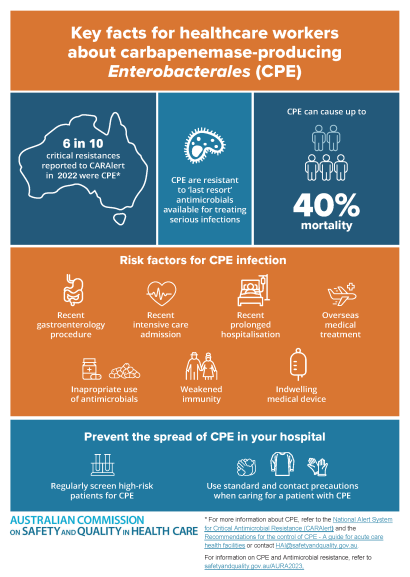
This infographic highlights the increasing risk of CPE in Australia, and the strategies health care providers can use to prevent and control the spread of CPE.
Hand hygiene eLearning modules to support healthcare workers in hand hygiene education
Learning modules for aged care workers and providers
The Aged Care Quality and Safety Commission provides learning modules to help aged care workers and providers understand their obligations and supply safe, high-quality care.