National guidance for Clinical Quality Registries
Australia’s national Clinical Quality Registries (CQRs) make a unique contribution to the Australian health system. They collect, analyse and report information about the care and outcomes being delivered by health service organisations, and serve as a fundamental driver of ongoing improvements in the safety and quality of the care provided to Australian consumers.
Australian Framework
Working with an Advisory Group, including CQR experts and representatives from all states and territories, the Commission has revised the Framework for Australian Clinical Quality Registries 2014 to produce the Australian Framework for National Clinical Quality Registries 2024 (the Framework). The Framework has also been informed by a national consultation process with a broad range of stakeholders.
The Framework supports CQRs in collecting, analysing, and reporting clinical data, to maximise the value of Australia’s clinical quality outcomes data. It aligns with the Australian Government’s National Strategy for Clinical Quality Registries and Virtual Registries 2020–2030 ultimately leading to better patient outcomes across Australia.
What’s new about the Framework
- Emphasises the role CQRs play in driving improvements across the health system
- Practical guidance on CQR governance
- Quality Standard for CQRs and self-assessment checklists
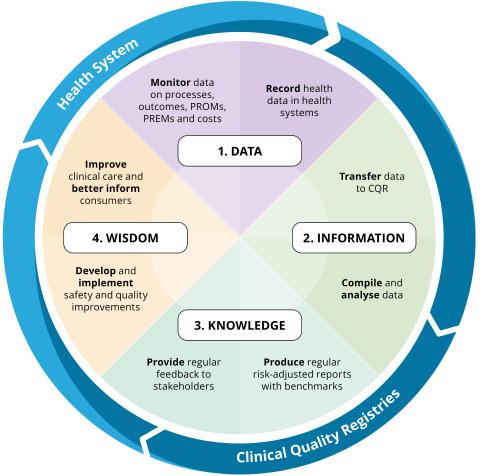
CQR feedback loop
The CQR feedback loop for ongoing improvements in the safety and quality of healthcare:
2023 consultation outcomes
Thank you to those who participated in the consultation process of the Framework for Australian clinical quality registries Second Edition - consultation version, and provided feedback. The Commission together with the CQR Framework Review Advisory Group has used the feedback to help refine the revised Framework. Access the feedback on the consultation below.
You can request a copy of the consultation version of the Framework for Australian clinical quality registries – Second Edition by emailing the Commission via CQR@safetyandquality.gov.au.
Australian Register of Clinical Registries
The Commission has developed the Australian Register of Clinical Registries (ARCR) to facilitate collaboration and awareness of registry activity among key stakeholders.
The ARCR is voluntary and contains a list of clinical registries that have self-nominated to be published on the register. Once a clinical registry is registered via the online form, Commission staff will contact the registrant to confirm the information provided. A brief summary of the registry, web link and registry contact details will be published on the Commission’s website.
For clarification, the registration process does not include approval or endorsement by the Commission that the clinical registry is a clinical quality registry.
Other tools and resources
More information
- Background on the Framework for Australian Clinical Quality Registries
The Framework is part of a national body of work to help CQRs establish and maintain systems and processes to achieve their core purpose of contributing to a learning health system that will drive better health care and patient outcomes.
- Prioritised list of clinical domains project
The Commission implemented a process to create a prioritised list of clinical domains for potential development of national clinical quality registries.
If you have any questions on the project and associated consultation please contact the project team via email at CQR@safetyandquality.gov.au
If you would like to be kept up-to-date on the clinical quality registries, register your interest to receive updates.








