Australian Unique Device Identifier Framework
Australia is establishing a unique identification system for implantable medical devices.
The Commission, in collaboration with the Therapeutic Goods Administration (TGA) and the Australian Government Department of Health is undertaking a project to develop and pilot the Australian Unique Device Identifier Framework (UDI Framework) for Australian health service organisations (UDI4H).
Implementation of the UDI4H Framework aims to improve patient safety by enhancing capability to effectively respond to adverse events, support recalls, and build consumer confidence in Australia’s system for post-market surveillance. It will also improve efforts by jurisdictions, and the private sector, to identify safety or performance concerns.
Queensland Health has joined the Early Adopter Project for UDI4H and will work with the Commission and TGA to develop the initial UDI4H Framework with resources which will allow other jurisdictions to pilot and implement the UDI4H into the future.
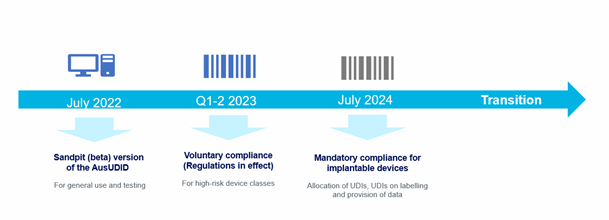
UDI4H Early Adopter Project Stages

If you would like further information and resources on implementation of the UDI4H Framework please contact the team at UDI4H@safetyandquality.gov.au
Background
In October 2020, the Australian Government announced that it would strengthen patient safety through the establishment of an Australian UDI system for medical devices. In February 2021, changes were made to the Therapeutic Goods Act to provide for the collection of the UDI data in an Australian UDI database, the creation of regulations to support the collection of data, and the changes required to device labels. The Therapeutic Goods Administration is establishing and will maintain the supporting infrastructure (the AusUDID database and data collection, and regulations around labelling requirements).
To find out more information regarding the progress of the national UDI implementation please go to:
The TGA as the Regulator for Medical Devices has the following implementation timeline.