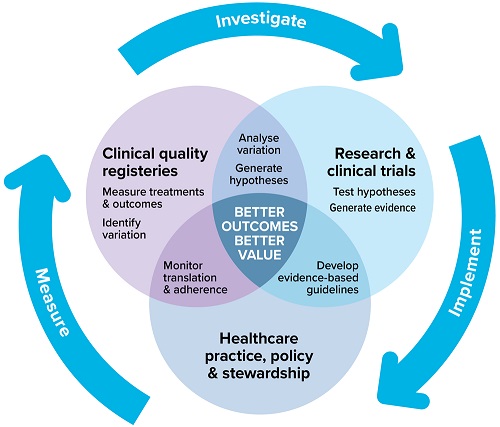
Resources for the National Clinical Trials Governance Framework
The Commission has developed resources for health service organisations (HSOs), accrediting agencies and assessors to support implementation of the safety and quality standards of the National Clinical Trials Governance Framework (NCTGF).
Key messages
The Commission are constantly working on delivering new and updated resources to support the sector in implementing the NCTGF. Recent releases and current projects include:
- A new Advisory CT25/01 on Extension of the Maturity Rating Scale for Clinical Trial Service Assessments against the National Clinical Trials Governance Framework (released Sept 2025)
- An update to Advisory AS18/01 Advice on not applicable actions that includes the NCTGF (released Sept 2025)
- An assessment outcomes data dashboard (released July 2025)
- A fact sheet on Incident Management (released July 2025)
- A new webinar series in development for early 2026
- Enhancements to the self-assessment and monitoring tools of the Clinical Trials Portal. Feedback on development opportunities can be shared directly with the Health and Medical Research Team via HMR@safetyandquality.gov.au
Download the Framework and User Guide
Knowledge building resources
Fact sheets and advisories
Fact sheets
These fact sheets provide details on the process and scope of assessment to the NCTGF.
Advisories
An Advisory is a formal communication issued by the Commission to accrediting agencies and health service organisations. Advisories provide guidance and direction on the interpretation and/or assessment of the NCTGF under the AHSSQA Scheme. Advisories will usually be issued when the Commission identifies that additional support is required to assist with the consistent implementation or assessment of a specific action or topic. An advisory may also be issued where the implementation of an action requires a stepwise process with long implementation timeframes.
Roles and responsibilities
Understanding the roles and functions of identified positions relating to clinical trial service provision within a health service is an important step in preparing for accreditation to the NCTGF.
For example, Action 1.1 of the NCTGF requires health service organisations to ensure roles and responsibilities are clearly defined for the governing body, management, clinicians and the workforce in relation to the provision of clinical trial services. The following fact sheets are intended to support health service organisations meet this requirement.
Webinars
Discover more about the NCTGF and the applicable NSQHS Standards through the Commission's webinar series. Hear reflections from health service organisations who have been through accreditation and learn as they describe the process of assessment and how they prepared for it.
| Webinar | Overview |
|---|---|
| What is the National Clinical Trials Governance Framework? June 2020 |
If you work in clinical trials, this video provides you with useful information on the implementation of the NCTGF and will help you understand how to prepare for accreditation |
| Introduction to and application of the NSQHS Standards to the Governance Framework July 2024 |
Are you implementing the National Clinical Trials Governance Framework within your HSO for clinical trial services? This webinar series provides an overview of the NCTGF and encompassing NSQHS Standards, along with reflections from HSOs that describe the assessment process and activities undertaken to prepare. |
| Standard 1: Clinical Governance and Standard 2: Partnering with Consumers Standards with case studies July 2024 |
This webinar includes a deeper look at the core NSQHS Standards which make up the NCTGF, specifically the Clinical Governance and Partnering with Consumers Standard, case studies from HSOs, insights from a rural and remote health service on activities to engage First Nations populations in clinical trial services and reflections from the private sector on the process of accreditation. |
| Tools for streamlining Governance Framework November 2024 |
Learn about resources to support the integration of applicable actions under the NCTGF, including:
|
| Behind the scenes: the Governance Framework in action November 2024 |
Hear directly from a national private hospital network that participated in the pilot program. They share updates on their current status with implementation, as well as the related opportunities and challenges. Learn how large public and private metropolitan HSOs who have received established ratings under the maturity scale approached implementation within their service contexts for clinical trial service provision. |
| National Clinical Trials Governance Framework - Process of assessment and resources April 2025 |
In the first webinar in the latest series hear insights into the assessment process from an experienced assessor and from the observations of independent industry consultants. Learn about resources currently under development by the Commission to support the sector in implementing the NCTGF. |
| National Clinical Trials Governance Framework - Exemplar assessments in practice April 2025 |
In the second webinar in the latest series hear reflections on the challenges and opportunities of implementation of the NCTGF from large metropolitan and regional health services with diverse patient populations who have successfully undergone assessment. Also learn about de-centralised clinical trial models, including teletrials and how HSOs using these models are working to implement the NCTGF. |
Exemplar practice
Health services organisations considered to demonstrate exemplar practices are invited to share their experiences through the Commission's public webinars. Exemplar practices are real-world examples of health service organisations conducting clinical trial services that demonstrate best practice in the implementation of the NCTGF.
Please contact HMR@safetyandquality.gov.au to discuss opportunities for recognising exemplar practice.
Fact sheets and advisories
Fact sheets
These fact sheets provide details on the process and scope of assessment to the NCTGF.
Advisories
An Advisory is a formal communication issued by the Commission to accrediting agencies and health service organisations. Advisories provide guidance and direction on the interpretation and/or assessment of the NCTGF under the AHSSQA Scheme. Advisories will usually be issued when the Commission identifies that additional support is required to assist with the consistent implementation or assessment of a specific action or topic. An advisory may also be issued where the implementation of an action requires a stepwise process with long implementation timeframes.
Roles and responsibilities
Understanding the roles and functions of identified positions relating to clinical trial service provision within a health service is an important step in preparing for accreditation to the NCTGF.
For example, Action 1.1 of the NCTGF requires health service organisations to ensure roles and responsibilities are clearly defined for the governing body, management, clinicians and the workforce in relation to the provision of clinical trial services. The following fact sheets are intended to support health service organisations meet this requirement.
Webinars
Discover more about the NCTGF and the applicable NSQHS Standards through the Commission's webinar series. Hear reflections from health service organisations who have been through accreditation and learn as they describe the process of assessment and how they prepared for it.
| Webinar | Overview |
|---|---|
| What is the National Clinical Trials Governance Framework? June 2020 |
If you work in clinical trials, this video provides you with useful information on the implementation of the NCTGF and will help you understand how to prepare for accreditation |
| Introduction to and application of the NSQHS Standards to the Governance Framework July 2024 |
Are you implementing the National Clinical Trials Governance Framework within your HSO for clinical trial services? This webinar series provides an overview of the NCTGF and encompassing NSQHS Standards, along with reflections from HSOs that describe the assessment process and activities undertaken to prepare. |
| Standard 1: Clinical Governance and Standard 2: Partnering with Consumers Standards with case studies July 2024 |
This webinar includes a deeper look at the core NSQHS Standards which make up the NCTGF, specifically the Clinical Governance and Partnering with Consumers Standard, case studies from HSOs, insights from a rural and remote health service on activities to engage First Nations populations in clinical trial services and reflections from the private sector on the process of accreditation. |
| Tools for streamlining Governance Framework November 2024 |
Learn about resources to support the integration of applicable actions under the NCTGF, including:
|
| Behind the scenes: the Governance Framework in action November 2024 |
Hear directly from a national private hospital network that participated in the pilot program. They share updates on their current status with implementation, as well as the related opportunities and challenges. Learn how large public and private metropolitan HSOs who have received established ratings under the maturity scale approached implementation within their service contexts for clinical trial service provision. |
| National Clinical Trials Governance Framework - Process of assessment and resources April 2025 |
In the first webinar in the latest series hear insights into the assessment process from an experienced assessor and from the observations of independent industry consultants. Learn about resources currently under development by the Commission to support the sector in implementing the NCTGF. |
| National Clinical Trials Governance Framework - Exemplar assessments in practice April 2025 |
In the second webinar in the latest series hear reflections on the challenges and opportunities of implementation of the NCTGF from large metropolitan and regional health services with diverse patient populations who have successfully undergone assessment. Also learn about de-centralised clinical trial models, including teletrials and how HSOs using these models are working to implement the NCTGF. |
Exemplar practice
Health services organisations considered to demonstrate exemplar practices are invited to share their experiences through the Commission's public webinars. Exemplar practices are real-world examples of health service organisations conducting clinical trial services that demonstrate best practice in the implementation of the NCTGF.
Please contact HMR@safetyandquality.gov.au to discuss opportunities for recognising exemplar practice.